T cell immunity and immunosuppression in cancer
The immune system has a powerful ability to recognize and kill cancer cells, but its function is often suppressed within tumours, preventing clearance of disease. Understanding how tumors suppress immunity has led to major therapeutic advances, including checkpoint inhibitors targeting PD-1 and CTLA-4. However, most patients do not durably respond to today’s immunotherapies.
Our laboratory aims to discover how cancers suppress T cell responses to evade destruction and resist immunotherapy. We aim to translate fundamental discoveries into new more effective therapies for patients with cancer, combining mechanistic immunology with cutting-edge approaches including CRISPR-based functional genetics and directed tumor evolution. Our research is increasingly focused on developing novel biologic, cellular and molecular therapies to improve treatment of advanced cancer patients or to prevent cancer recurrence in those at high risk of metastasis.
Suppression of T cell immunity within tumours
Tumors create hostile cellular and chemical environments that suppress anti-tumor immunity.
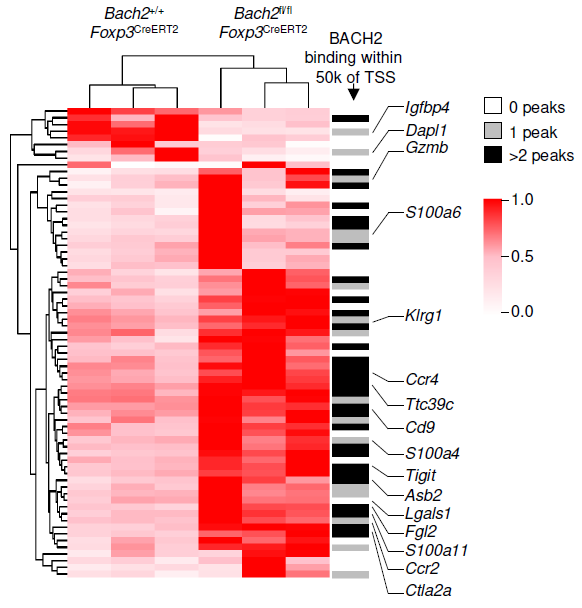
Regulatory T (Treg) cells suppress conventional T cell responses and are a major barrier to anti-tumor immunity (Figure 1). We are interested in understanding how Treg cells develop and function. We discovered that the transcriptional repressor BACH2 is essential for Treg cell development and established a widely-accepted molecular model for BACH2 function in lymphocytes (Roychoudhuri et al., Nature 2013; Roychoudhuri et al., Nature Immunology 2016). This work explained why genetic variations at the human BACH2 locus confer susceptibility to autoimmunity and allergy, and led to identification—through international collaboration—of BACH2-related Immunodeficiency and Autoimmunity (BRIDA), a previously unrecognised human monogenic disease, improving clinical management for affected patients worldwide (Afzali et al., Nature Immunology 2017). We showed how a distal enhancer at the prominent 11q13.5 autoimmune disease risk locus restricts gut inflammation by promoting expression of the TGF-β docking receptor GARP on Treg cells (Nasrallah et al., Nature 2020).
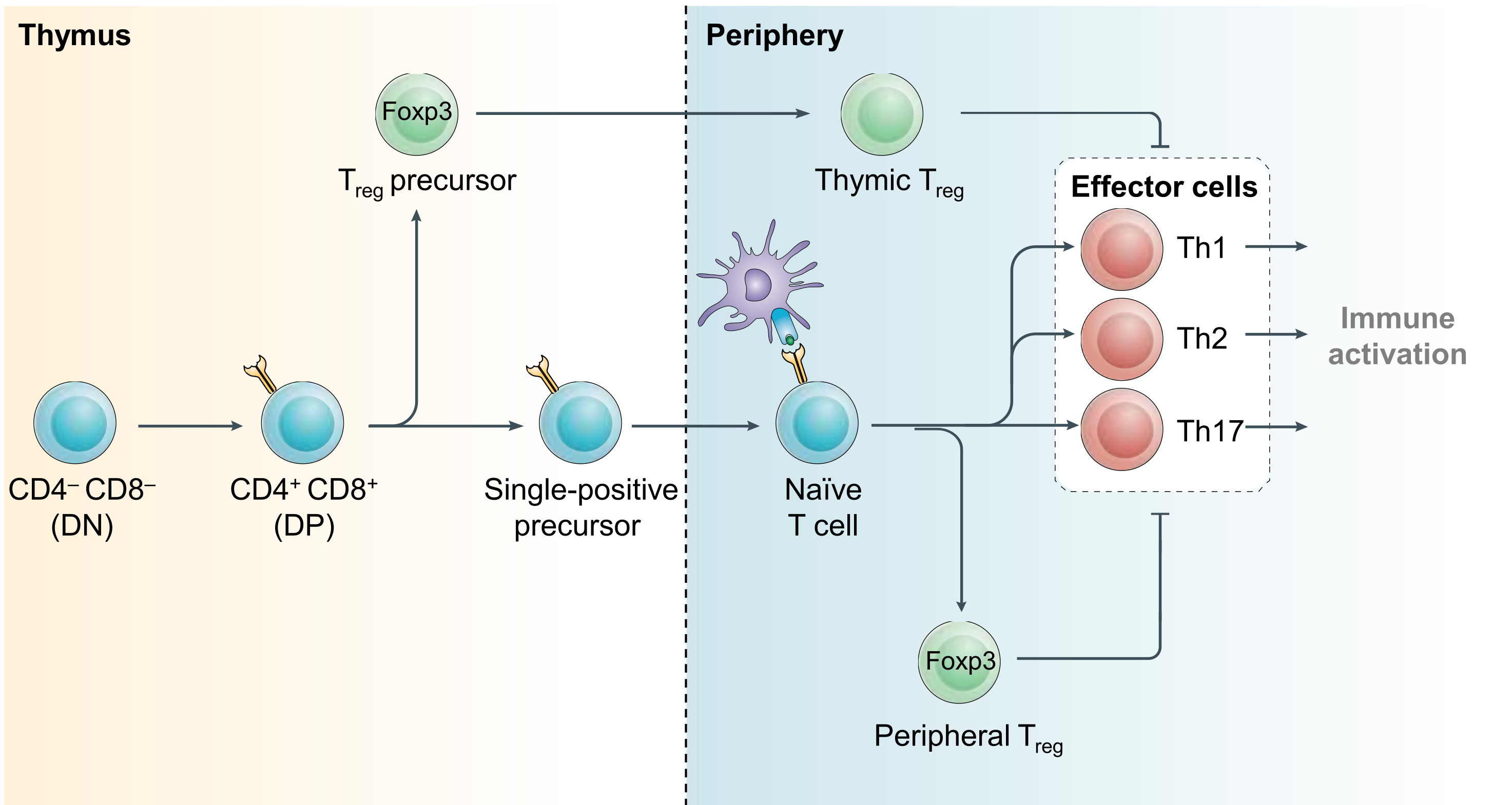
Figure 1. Regulatory T cell development. CD4+ effector and regulatory T (Treg) cells arise from common precursor cells within the thymus and periphery by exert opposing functions. Treg-mediated restraint of effector cell function is a critical immunoregulatory mechanism required to prevent lethal inflammation. Modified from Igarashi, Kurosaki and Roychoudhuri, Nat Rev Immunol 2017.
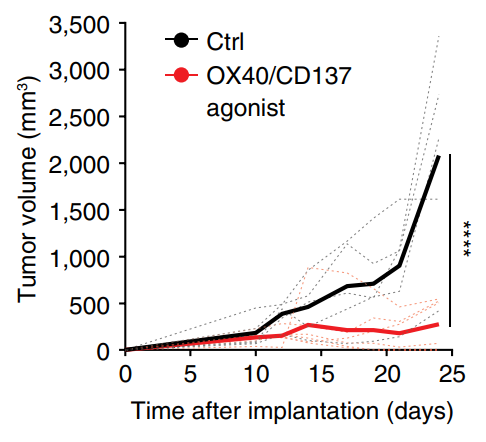
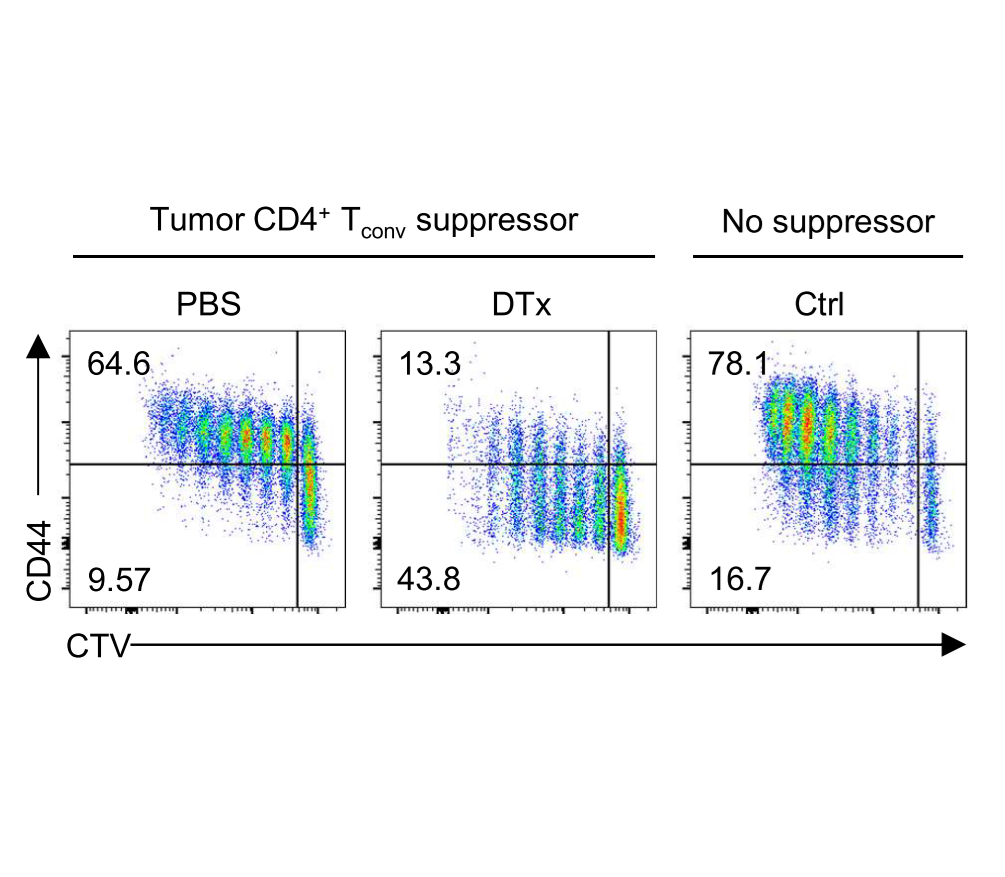
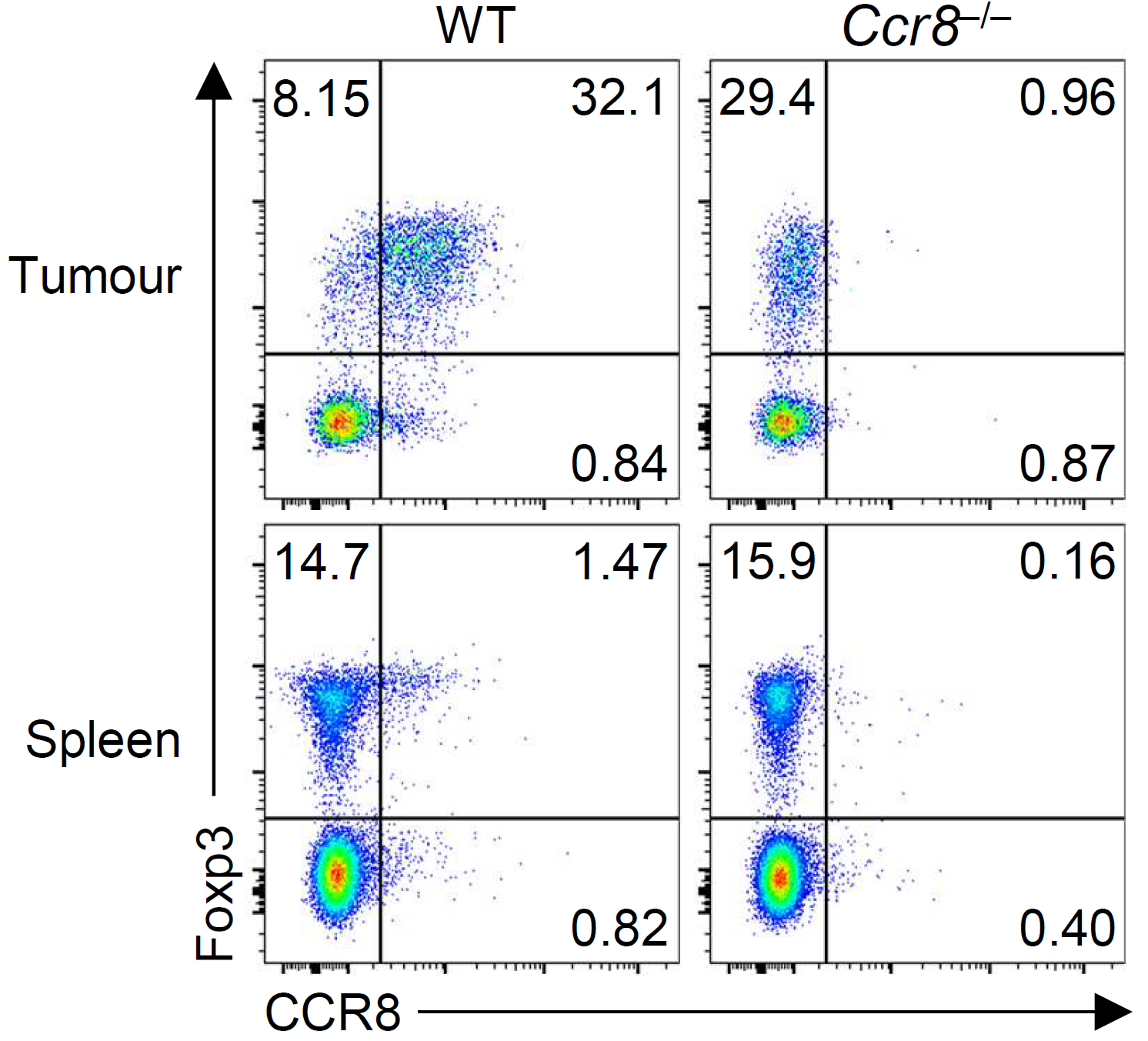
Within tumors, we discovered that activated Treg responses are maintained by quiescent stem-like progenitor cells, raising questions about how Treg populations are maintained within tumours (Grant et al., J Exp Med 2020). We identified CCR8 as a marker of highly suppressive tumor-infiltrating Treg cells and a distinct suppressive subset of conventional CD4+ T cells that mediate immunotherapy resistance through IL-10 production (Whiteside et al., Science Immunology 2023). We are now examining how therapeutic strategies could reprogram Treg cells within tumors, including OX40/CD137 bispecific antibodies that convert Tregs into IFN-γ-producing cells that enhance anti-tumor immunity (Imianowski et al., Cancer Res Commun 2024).
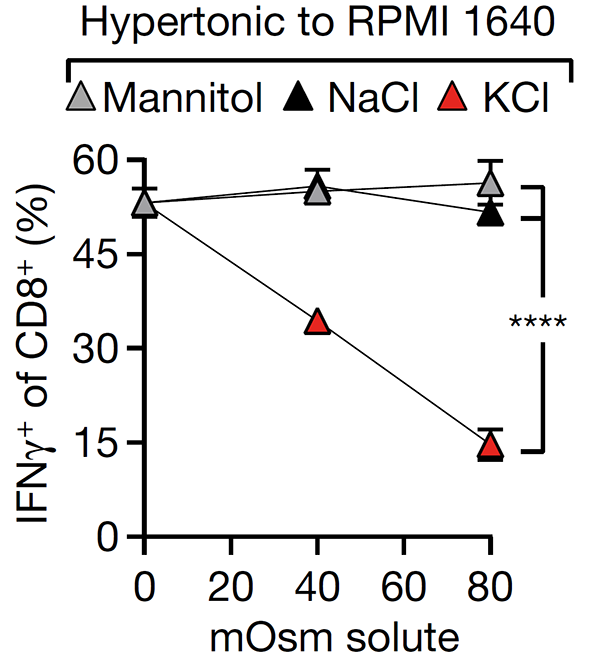
We are interested in identifying and targeting mechanisms by which the tumor microenvironment disables T cell responses. We discovered that dying tumor cells release intracellular potassium that suppresses T cell activation (Eil et al., Nature 2016) and that tumor acidity inactivates interleukin-2, enabling us to engineer pH-selective IL-2 variant with preferential activity in acidic tumors (Gaggero et al., Science Immunology 2022). We are now applying directed tumor evolution and high-throughput CRISPR screens to identify additional immunoregulatory mechanisms within tumors, with the goal of developing next-generation biological and small molecule approaches to enhance T cell function.
T Cell Maintenance and Dysfunction
Effective immune responses to cancer require T cells to persist over long periods while retaining their ability to kill tumor cells. We have studied the molecular programs that control T cell longevity and how tumors induce T cell dysfunction—insights we are now aim to translate into improved cell therapies for cancer patients (Figure 2).
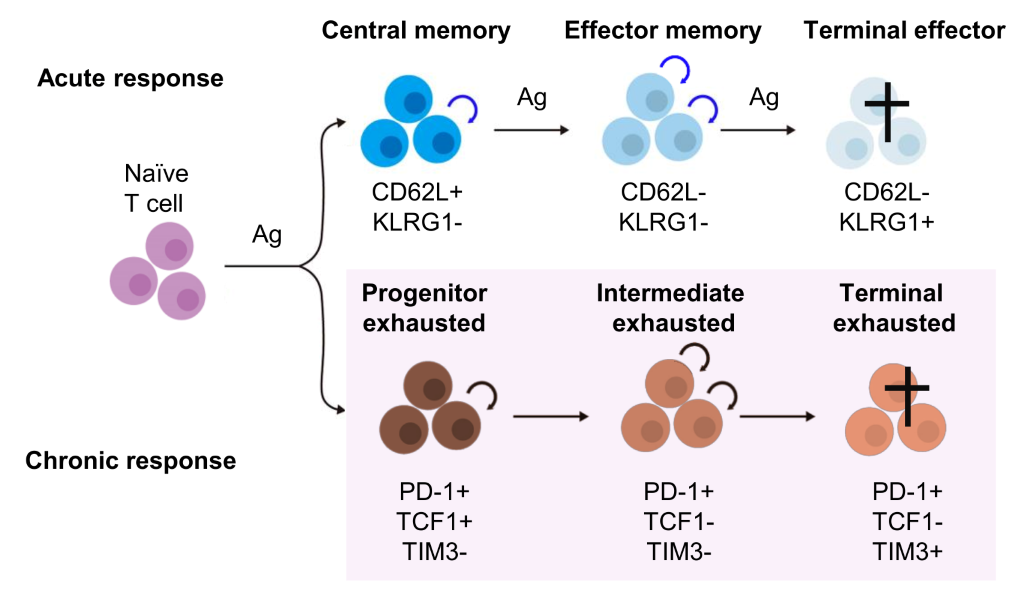
Figure 2. Maintenance of acute and chronic T cell responses. In both acute and chronic immune responses, T cells undergo progressive differentiation accompanied by acquisition of effector functions and loss of maintenance potential self-renewal and multipotency. This loss of maintenance potential is a key feature of the progression of T cells from naive, effector, and memory T cell subsets in acute responses, as well as progenitor, intermediate, and terminally exhausted T cells in chronic responses.
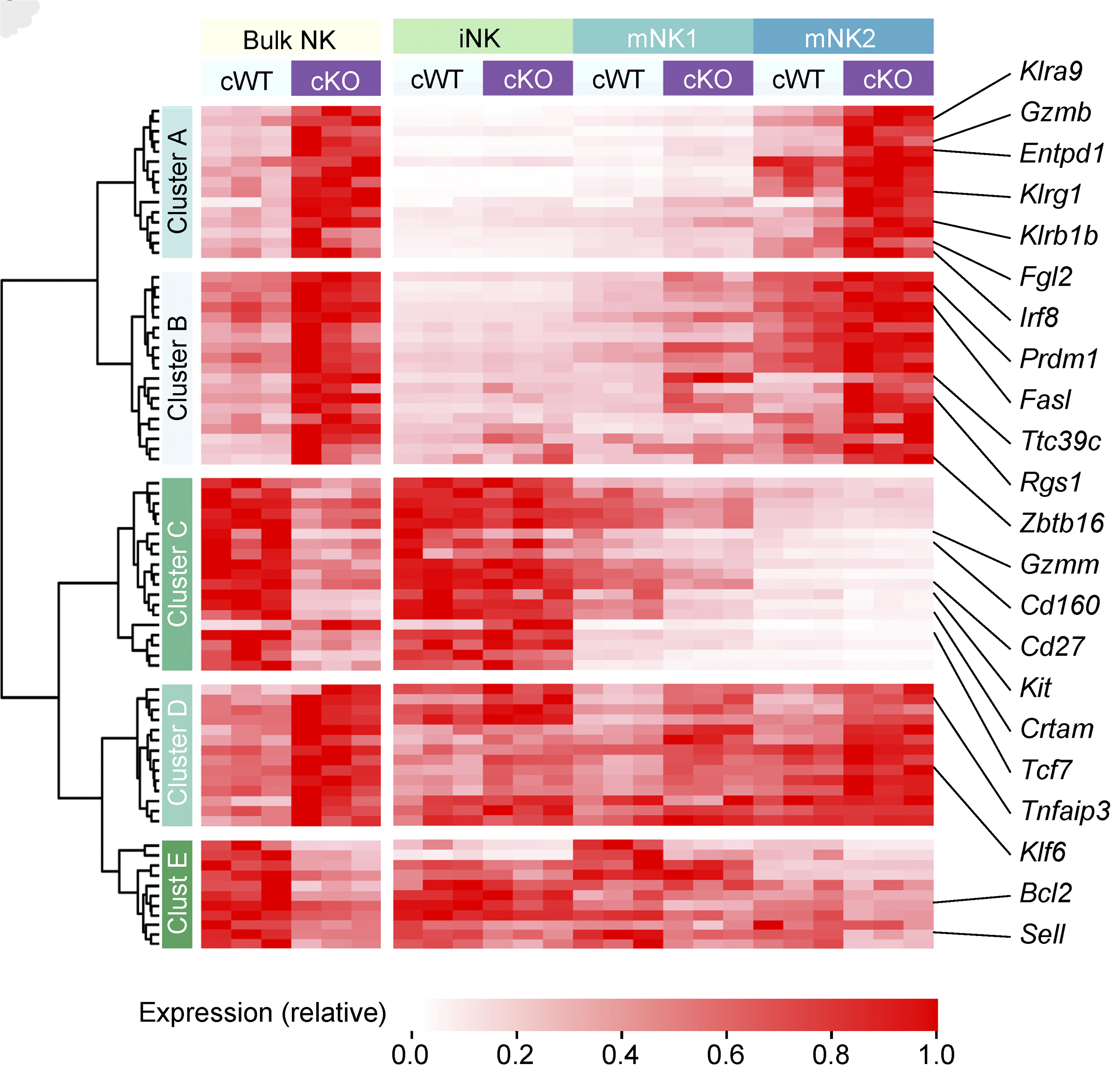
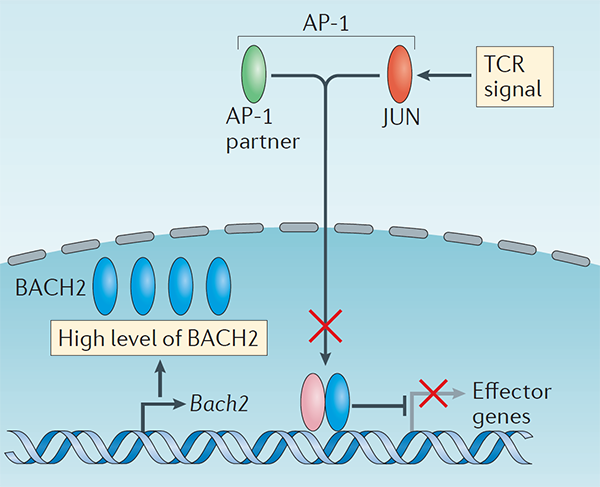
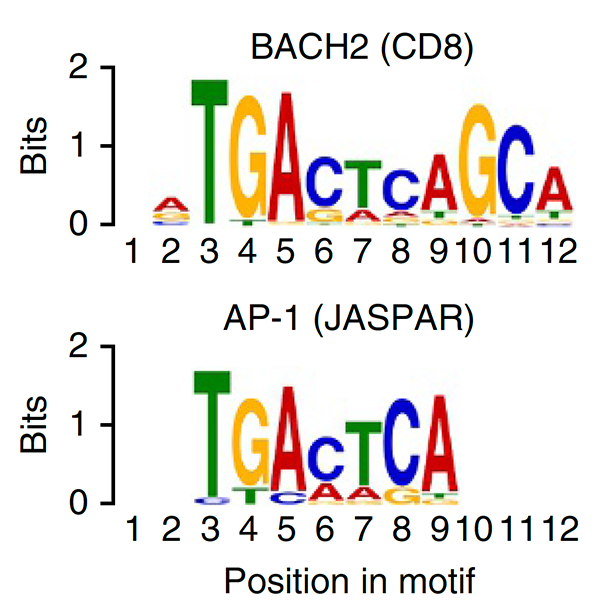
We conducted one of the earliest multiplexed single-cell gene expression analyses of immune cells, revealing heterogeneity in memory CD8+ T cell responses to vaccination (Flatz, Roychoudhuri et al., PNAS 2011) and defining the transcriptional and epigenetic programs of vaccine-induced memory T cells (Roychoudhuri et al., Vaccine 2015). We discovered that BACH2 functions as a regulator of T cell quiescence, promoting long-lived memory CD8+ T cell responses to viral infection (Roychoudhuri et al., Nature Immunology 2016). Mechanistically, BACH2 represses T cell receptor-driven effector programs by occupying AP-1 binding sites and blocking access by AP-1 transcription factors. This quiescence program extends beyond CD8+ T cells: we showed that long-term Treg cell maintenance depends on a quiescent subset marked by high Bach2 expression (Grant et al., J Exp Med 2020), and that BACH2 similarly constrains NK cell maturation, restricting NK-mediated immunosurveillance of lung metastasis (Imianowski et al., J Exp Med 2022). We contributed to studies showing that AKT pathway inhibition generates T cells with enhanced memory characteristics and superior anti-tumor activity in adoptive cell therapy (Crompton et al., Cancer Res 2015), and that memory T cell-driven differentiation can impair therapeutic efficacy (Klebanoff et al., J Clin Invest 2015).
We are now using these insights to engineer improved CAR T cell therapies. Multiple ongoing projects aim to harness T cell maintenance programs to generate cell products with enhanced persistence and anti-tumor function for patients with advanced cancers.
Immune Interception of Early Cancer and Cancer Metastasis
Advanced cancers are protected by a well-established immunosuppressive microenvironment that limits the efficacy of current immunotherapies. Yet early pre-malignant cancers and newly established or dormant micrometastases lack the highly immunosuppressive microenvironment of established tumours (Fig. 3). This creates an opportunity for therapies that utilize the immune system to prevent cancer metastasis in patients with early-stage cancer at risk of recurrence, or to prevent early cancer in high-risk individuals.
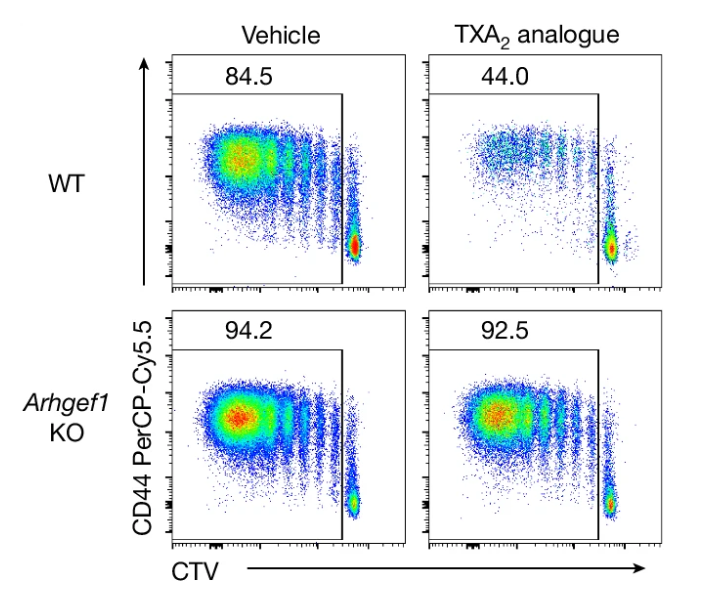
We found that aspirin prevents cancer metastasis by blocking platelet-derived thromboxane A2 (TXA2), which suppresses T cell immunity (Yang et al., Nature 2025). This work provides a mechanistic understanding of aspirin’s previously observed anti-metastatic activity. Building on this interest, we are developing preventative immunotherapies—including novel biologics and small molecules—to prevent cancer or its recurrence in high-risk patients. By targeting cancer before widespread metastatic dissemination occurs, we aim to improve outcomes for patients facing the greatest cancer burden.
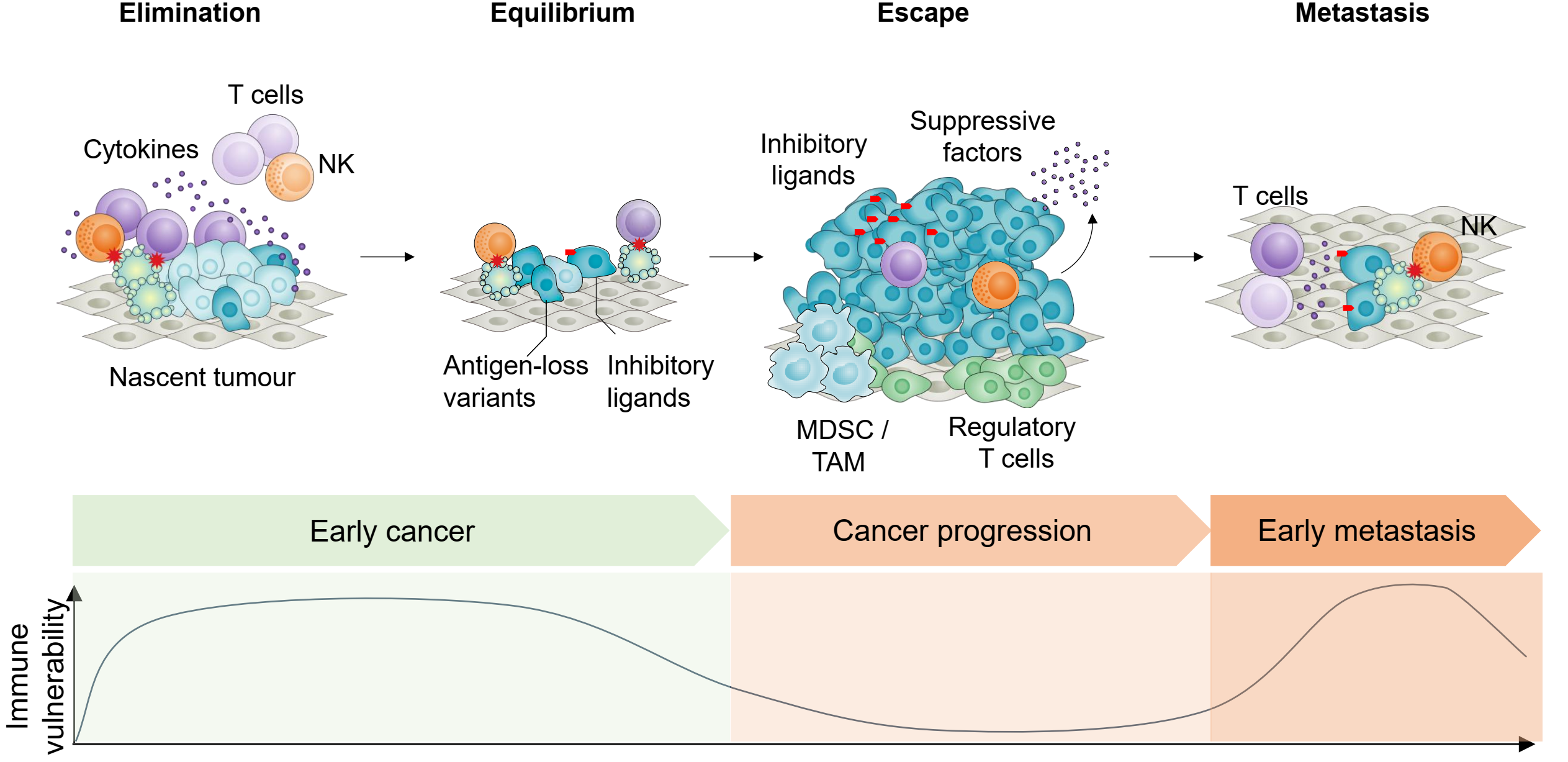
Figure 3. Immune vulnerability of cancer cells at distinct stages of cancer development provides a potential window for immune prevention. Cancer progression through elimination, equilibrium, and escape phases results in the establishment of a protective immunosuppressive microenvironment, featuring regulatory T cells, MDSCs, TAMs, and suppressive factors that shield advanced tumours from immune attack. However, early-stage cancers and disseminated micrometastases lack this immunosuppressive armor, remaining vulnerable to elimination by T cells and NK cells. The immune vulnerability curve (bottom) reveals two critical therapeutic windows: early cancers before escape mechanisms develop, and micrometastatic deposits that are deprived of the protective tumour microenvironment. This vulnerability of early and disseminated disease represents a major opportunity for preventive immunotherapy—targeting cancer when it is most susceptible to immune elimination rather than after immunosuppressive defenses are established.
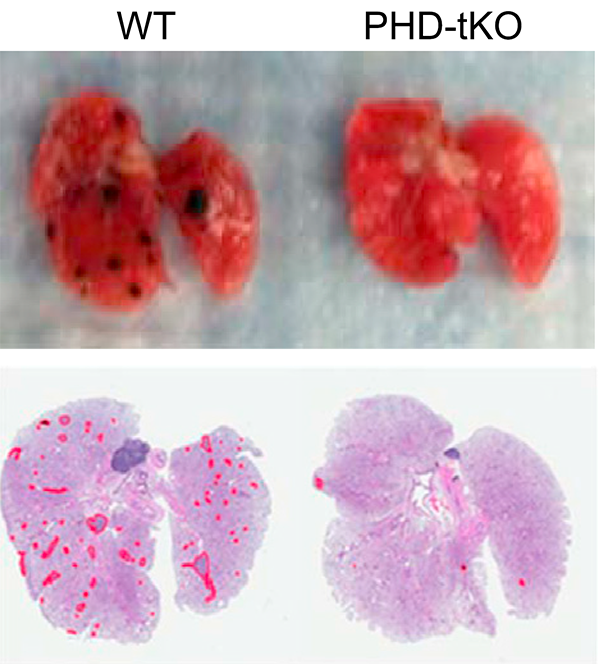
We showed that T cells sense high oxygen concentrations in lung tissue through prolyl hydroxylase proteins, establishing an immunologically tolerant niche vulnerable to metastasis (Clever et al., Cell 2016). We demonstrated that NK cell maturation states determine metastatic susceptibility, with BACH2 limiting NK-mediated immunosurveillance of lung metastases (Imianowski et al., J Exp Med 2022).
We welcome inquiries from funders interested in supporting our fundamental and translational cancer immunology research and from talented scientists seeking to join our team.
Research Highlights
(For a full list of publications see below)
Group Members
[Team] [Join us]@RoychoudhuriLab
Collaborators:
- Klaus Okkenhaug (Pathology)
- David Adams (Sanger)
- Adrian Liston (Babraham)
- Enrico Lugli (Humanitas, Milan)
- Tim Halim (CRUK CI)
- Suman Mitra (INSERM Lille)
- Gosia Trynka (Sanger)
Environment:
- Department of Pathology (Cambridge)
- CRUK Cambridge Centre (Cambridge)
- Babraham Institute (Cambridge)